Review of the current state of the clinical trials environment
Sapere provided extensive analytical support to a consortium of researchers from the University of Auckland and the University of Otago on a Health Research Council (HRC) and Ministry of Health funded research project. The consortium was brought together to review Aotearoa New Zealand’s clinical trials activity and infrastructure. Sapere worked with the primary investigators to review the current state of the clinical trials environment and set out what infrastructure and capabilities would be required to enable a sustainable, effective, and equitable national clinical trials system.
This project was undertaken over the span of a year and required Sapere, alongside the consortium, to engage on many occasions with a wide range of stakeholders with varying perspectives, lived experiences, expertise, and priorities to understand what any future infrastructure needed to do to best serve the people within the system. The project undertaken by the consortium was in two parts:
- A description and review of the current state of clinical trials infrastructure and activity in Aotearoa New Zealand, to establish the ‘lay of the land’ of clinical trials infrastructure and activity in Aotearoa New Zealand. This included forming a picture of what was working well, what was not working well, and where there were gaps and opportunities to enhance the system to enable equitable participation and representation in clinical trials (as both participants and researchers amongst different groups).
- The development of a roadmap/options for clinical trials infrastructure based on best practice, stakeholder experience, and stakeholder voice, using what had been learned and captured through the current state analysis. The purpose of this roadmap was to inform an appropriate path forward, representative of what the group had heard from stakeholders and of what best practice can look like for a clinical trials system.
“This project was undertaken over the span of a year and required Sapere, alongside the consortium, to engage on many occasions with a wide range of stakeholders with varying perspectives, lived experiences, expertise, and priorities”
– Sapere
Sapere’s role in reviewing the current state
Sapere helped to facilitate over 70 interviews, a handful of workshops, and designed and analysed a survey that was responded to by over 350 diverse stakeholders with various roles and perspectives within the clinical trials system to form a picture on current state. At the request of the consortium, Sapere also conducted a scoping literature review of best practice clinical trials and research systems internationally to understand their strengths, and how the New Zealand system compared across all domains, ranging from data management and sovereignty to knowledge translation and implementation, through to indigenous voice in trials and trial design. A further literature review was conducted by Sapere on the value of clinical trials and health research more broadly, to strengthen the impetus for change.
Sapere’s role in development of a roadmap/options
Sapere helped to facilitate a World Café workshop to get people to think about essentials, from their perspective, for any new clinical trials system proposed. Seventy stakeholders had committed to attending the World Café workshop in person. Last-minute changes because of COVID-19 meant the workshop could no longer go ahead in person, and therefore the decision was made to run the workshop online. The same number of people attended the online workshop, and it was broadly a successful process, with only a few people dropping off throughout the day.
Following the World Café, Sapere helped the primary investigators to run a Delphi method survey to form some consensus on the absolutely essential components any proposed infrastructure must have (effectively getting stakeholders to agree upon different elements of clinical trials infrastructure in a process of prioritisation). The result of the Delphi method survey was a list of foundational principles that the consortium could put forward that should be underpin any future clinical trials infrastructure. The development of foundational principles allowed for some discretion and flexibility in the way the infrastructure is implemented.
The outcomes of the project
The figure below shows the visual culmination of all the learnings of the project, and what the consortium envisioned a successful clinical trials system could look like.
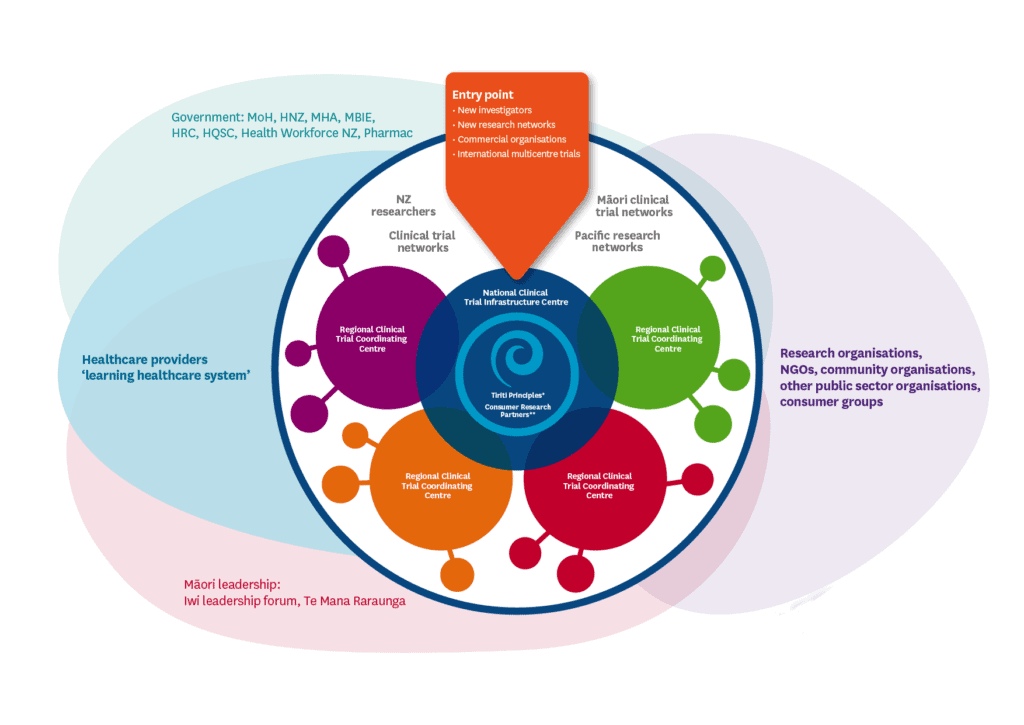
The research report explains in depth the themes, discussions, experiences, and views that underpin the need for change, and why the figure above looks the way it does. The report was accepted by the HRC and recognised by the Ministry of Health as an important piece of work in its bid to effect system-level changes in the wider health research space.[1]
[1] See: https://www.health.govt.nz/news-media/media-releases/health-agencies-working-together-harness-full-potential-clinical-trials.
[2] Final Report: https://cdn.auckland.ac.nz/assets/liggins/docs/HP8537%20-%20LIG_Clinical%20Trials_FINAL_v6.pdf
Our team included
- Dr Tom Love
- David Moore
- Tammy Hambling
- Kelvin Woock